CPRIT's $1.88 billion investment in basic, translational, clinical, and population research enhances Texas’ research prowess and boosts Texas’ national and international reputation in cancer research. CPRIT’s stable, substantial support is bringing preeminent research talent to the state and providing the catalyst for the development of new centers of cancer research excellence based at Texas institutions.

One Nobel Laureate

2 Lasker Award Recipients

41 members of National Academies

12 Howard Hughes Medical Institute Investigators

8 NCI Outstanding Investigator Awardees

12 NCI Specialized Programs of Research Excellence
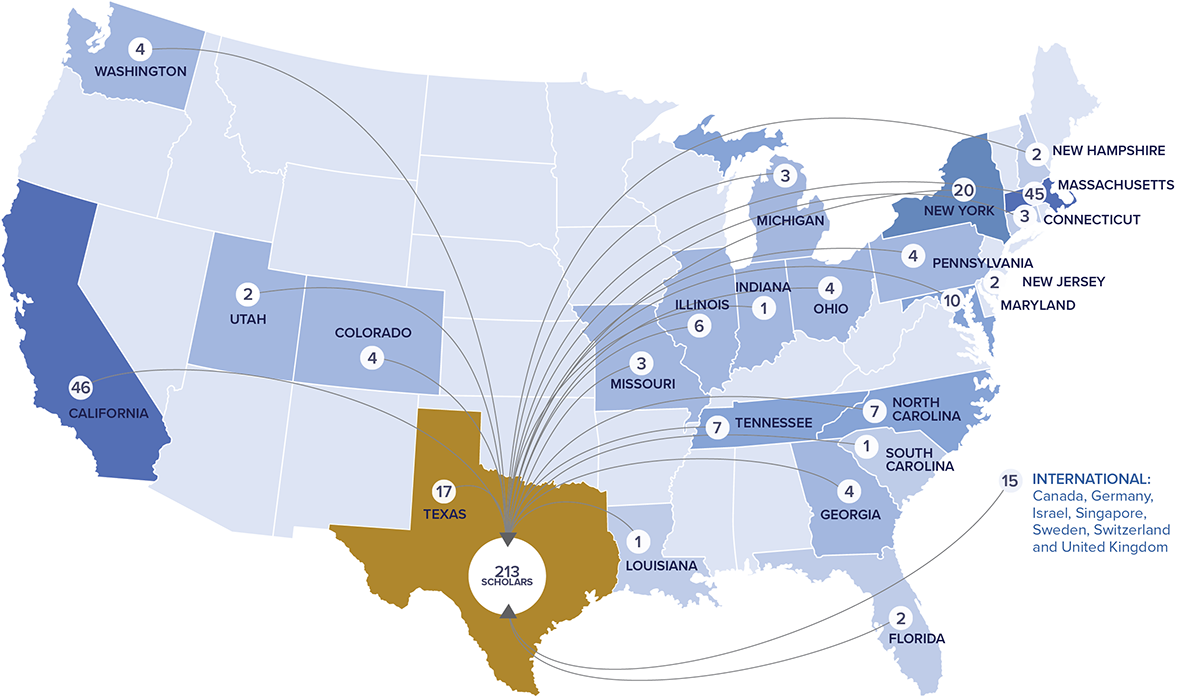
CPRIT’s unmatched recruiting program has successfully enhanced Texas’ long term cancer research efforts and increased the external visibility of the state in the medical and scientific communities. The 213 cancer researchers recruited to 21 institutions across Texas establish a critical mass of unmatched research talent in the state that will return dividends for decades to come.

Tanmay Lele, Ph.D., recruited to Texas A&M Engineering Experiment Station from the University of Florida with an Established Investigator grant approved in May 2020 (RR200043)
Dr. Lele’s work focuses on the role of mechanical forces in shaping a cell’s nuclear structure and the relationship of this to gene expression to answer a key question in cancer biology- why the nucleus is almost always abnormal in cancer cells. Dr. Lele, the Unocal Professor, Biomedical Engineering and Chemical Engineering at Texas A&M University, is further developing his methods that combine microscopy and advanced mathematical image analysis in partnership with cancer scientists at the Texas Medical Center and members of the engineering/computer science faculty at Texas A&M to identify candidate therapies for medulloblastoma.

Caroline Ajo-Franklin, Ph.D., recruited to Rice University from the Lawrence Berkeley National Laboratory with an Established Investigator grant approved in November 2019 (RR190063)
Dr. Ajo-Franklin’s area of research is cutting edge – addressing the challenging issue of gastrointestinal cancer prevention and treatment from both a fundamental and applied perspective. Internationally recognized as a leader in the fields of synthetic biology and electromicrobiology, she has groundbreaking success in programing cells to communicate electrically with devices. Building on these innovations, Dr. Ajo-Franklin aims to create a new generation of affordable bioelectronic biosensors for cancer – “digital pill” devices that merge conductive materials and biological electronics for dynamic sensing within the gastrointestinal system.

Suzanne D. Conzen, M.D., recruited to The University of Texas Southwestern Medical Center from the University of Chicago with an Established Investigator grant approved in May 2019 (RR190037)
Dr. Conzen, Chief of the Division of Hematology and Oncology at UT Southwestern, came to Texas with a mission to initiate breast and prostate cancer clinical trials with several faculty members during her first week on the job. Soon after arriving, UT Southwestern named her a co-leader of the prostate cancer Specialized Program of Research Excellence (SPORE) application. SPORE grants, funded by the National Cancer Institute, are among the most comprehensive multidisciplinary research mechanisms available for translational research in an NCI-designated cancer center. Her evidence-based, multidisciplinary approach to cancer risk and treatment considerations for vulnerable populations complement the UT Southwestern Simmons Cancer Center’s increasing efforts to reach underserved communities with novel cancer prevention and treatment strategies in the context of community-based participatory research.

Gloria Echeverria, Ph.D., recruited to Baylor College of Medicine with a First-Time, Tenure-Track Faculty Member Award approved in November 2019 (RR200009)
Although early in her career, Dr. Echeverria has already distinguished herself through impactful basic and translational cancer research combined with an ability to consistently obtain independent funding. The Echeverria laboratory at Baylor College of Medicine studies the molecular evolution of triple negative breast cancer (TNBC) therapy resistance and metastasis. Given her remarkable record marked by important publications, funding, and numerous awards, she is an upcoming leader in the fight against breast cancer. The publication The Scientist named Dr. Echeverria a “Scientist to Watch” in December 2020.

Klementina Fon Tacer, DVM, Ph.D., recruited to the Texas Tech University School of Veterinary Medicine in Amarillo from St. Jude Children's Research Hospital in Memphis, Tennessee with a First-Time, Tenure Track Faculty Member Award approved in May 2020 (RR200059)
Dr. Fon Tacer, a member of the new veterinary school’s inaugural faculty, is researching novel mechanisms that protect mammalian germ cells against stress and how cancer co-opts those mechanisms. Her goal is advancing cancer treatment and preserving the fertility of childhood cancer survivors. Dr. Guy Loneragan, dean of the School of Veterinary Medicine, explained how important Dr. Fon Tacer’s recruitment was to the school, “Dr. Fon Tacer is going to change lives for generations to come…Her research is transformative and will impact countless Texans in the years ahead. I am so thankful to the CPRIT Oversight Committee for awarding Texas Tech this recruitment award to make it possible to bring Dr. Fon Tacer to Texas and to the School of Veterinary Medicine. Her work in comparative oncology will save lives.”

Theresa Guise, M.D., recruited to The University of Texas MD Anderson Cancer Center from Indiana University School of Medicine with an Established Investigator award approved in November 2019 (RR190108)
Dr. Guise is a leading physician scientist in the study of skeletal complications of cancer, including bone metastasis, bone loss and muscle weakness. She has received multiple honors and awards in recognition of her contributions to bone research including an NIH Outstanding Investigator Award and a Komen Scholar Award. Her pioneering work helps to explain mechanisms by which metastatic tumors destroy bone and has contributed to FDA approved therapies for metastatic disease. When Dr. Guise accepted the recruitment award in January 2020, she became the 200th Scholar successfully recruited to Texas.
CPRIT is demonstrably making the state a national leader in cancer research. Evidence of this is in the increasing number of NCI designated cancer centers in Texas. By conferring its “Designated Cancer Center” status, the NCI recognizes an institution for its scientific leadership, resources, and depth and breadth of its research in basic, clinical, and population science.
CPRIT’s significant and sustained grant funding was a major factor assisting Texas institutions attain this preeminent status. Before CPRIT, Texas had one NCI Designated Comprehensive Cancer Center – The University of Texas MD Anderson Cancer Center. Now there are three. The University of Texas Southwestern Medical Center and the Baylor College of Medicine joined MD Anderson in this prestigious group. In addition, The University of Texas Health Science Center at San Antonio is an NCI-Designated Cancer Center.
Texans benefit through better access to clinical trials and advanced levels of cancer treatment not available through other health care providers. The enhanced standing of Texas’ cancer centers also elevates the state’s medical education and research efforts. Cancer center investigators are more competitive for research funding from NCI and other funding agencies and organizations. Research proposals from cancer center investigators account for three-quarters of the successful investigator-initiated grants awarded by NCI.







As scientific research technologies become more complex, cancer researchers increasingly require access to different types of specialized instruments and expertise. CPRIT’s Core Facility grants make cutting-edge technology available to multiple research institutions, crossing scientific disciplines and departments for a wide variety of cancer-related projects including laboratory, clinical, population-based, and computer-based research.
CPRIT-funded core facilities are hubs of innovation, connecting scientists with the tools and expertise that elevate their research projects. They are also an efficient and cost-effective way to leverage research expertise and specialized instrumentation. A typical core facility may include dedicated space, specialized scientific equipment, and expert staff. Core facilities may take many forms ranging from individual pieces of shared research equipment to large multi-center tissue databanks.
The network of 55 CPRIT-supported core facilities at 20 institutions across Texas provides scientists access to the most up-to-date technologies needed for cutting-edge cancer research. CPRIT’s dedicated investment in the core facility network in Texas is demonstrably enhancing the state’s research prowess at its academic institutions.
Awarded in May 2015 to The University of Texas Southwestern Medical Center (RP150596), $5,593,882
Directed by experts in computational biology and biostatistics, the Bioinformatics Core Facility provides clinical and basic cancer researchers with access to state-of-the-art computer hardware and software infrastructure for comprehensive biomedical data processing and analysis. The facility’s ability to integrate all biological information collected at UT Southwestern - from screens for cancer-driving genes to images of tumors and individual cancer cells to medical records that document the health history of a patient – enhances researchers’ understanding of cancer to better guide the detection, diagnosis, treatment and prevention of cancer.Awarded in August 2018 to Baylor College of Medicine (RP180785), $5,306,052
Building upon the success of its CPRIT-funded Texas Assistance for Cancer Cell Therapy (TACCT) core facility to move new therapies from the research laboratory into clinical trials, Baylor College of Medicine’s CARMIT provides cellular therapy products prepared under current Good Manufacturing Practices (cGMP) for the treatment of children with cancer. CARMIT expands the TACCT services by manufacturing and testing additional components, called vectors, which carry new genetic information into cells and improve the activity of cell therapies. The expanded core facility is an unsurpassed resource to Texas investigators researching childhood cancer treatments.Awarded in August 2020 to Texas Tech University Health Sciences Center in Amarillo (RP200572), $2,831,213
A state-of the-art imaging facility is indispensable for any competitive biomedical research institution. With the addition of three cutting-edge instruments (a confocal microscope system, an automated imager, and an instrument that is capable of whole animal live imaging), the CPRIT grant significantly expands the capabilities of the Jerry H Hodge School of Pharmacy’s existing imaging core facility.
Texas Tech University Health Sciences Center in Amarillo is one of only two facilities in the country with the confocal microscope, which allows scientists to look at details that are ten times smaller than wavelengths of visible light. These instruments greatly enhance the quality of obtainable data in cancer research projects. Their addition allows the schools of pharmacy, medicine and veterinary medicine to collaborate on advanced research.
Awarded in August 2019 to Texas A&M University Health Science Center Institute of Biosciences and Technology (RP190581), $4,690,019
The multi-institutional HtFCP Core Facility is a model for the power of collaborative research discovering the next generation of cancer therapeutics. The specialized high-throughput flow cytometers allow investigators to test the activity of thousands of drugs and drug combinations on cancer cells and immunotherapeutics. Serving Gulf Coast Consortium member institutions (Texas A&M Institute of Biosciences and Technology, Baylor College of Medicine, MD Anderson Cancer Center, University of Houston, The University of Texas Medical Branch - Galveston, The University of Texas Health Science Center at Houston, and Rice University), the HtFCP allows researchers to rapidly move promising discoveries from screening studies into clinical testing.Awarded in May 2015 to The University of Texas Health Science Center at Houston (RP150551), $5,277,338
Antibody therapy represents a breakthrough in combating cancer. Unlike small-molecule drugs, both the technological expertise and infrastructure for advancement of antibody leads are unavailable to most in the Texas cancer research community. This core facility provides access to the expertise necessary for advancement of antibodies from the basic research lab to drug candidates. Over the past five years, it has supported more than 30 Texas researchers to develop lead antibody candidates for cancer and generated an additional $19 million in drug development funding.Awarded in May 2015 to The University of Texas Health Science Center at San Antonio (RP150600), $3,277,895
Located at UT Health San Antonio’s Cancer Therapy and Research Center, the SBCC core facility provides cancer researchers with cutting-edge technology of single-cell isolation and analyses. The single-cell approach allows researchers to tease out differences between individual tumor cells that scientists may overlook when studying bulk tumors. The SBCC core facility provides researchers unprecedented opportunities to examine samples from cancer patients at the level of single cells, which may improve the clinical outcome for cancer patients through development of new diagnostic and prognostic tools.Research training programs supported by CPRIT grants develop the next generation of cancer researchers, increase the diversity of the cancer research workforce, and grow and sustain the state’s life science infrastructure. To date, 21 CPRIT-supported specialized training programs at nine institutions have educated 1,265 undergraduate students, graduate students, clinician trainees, and pre- and post-doctoral fellows.

CPRIT Trainee Andrew Gdowski, D.O., Ph.D.
The Osteopathic Scholars in Cancer Research (OSCR) training program at the University of North Texas Health Science Center at Fort Worth (RP170301)
The Osteopathic Scholars in Cancer Research training program led by Jamboor Vishwanatha, Ph.D., Regents Professor in the Graduate School of Biomedical Sciences at UNT Health Science Center, focuses on recruiting and training underrepresented minorities and disadvantaged students for the combined dual degrees of D.O. (physician) and Ph.D. (scientist) in cancer research.
There is a profound lack of diversity of physicians and physician scientists both in the U.S. and in Texas, highlighting a critical need to train more physicians with a commitment to reduce health disparities and train scientists actively working on cures for and improved diagnosis and treatment of cancer.
“The CPRIT OSCR program has provided me the support to complete the unique opportunity of training as a Doctor of Osteopathy physician-scientist at UNTHSC/TCOM. Training as a physician-scientist requires a great deal of institutional backing and mentorship support. My thesis project involved developing and testing bone targeted nanoparticles for treating metastatic prostate cancer which has potential implications for improving health disparities in prostate cancer patients. Through the OSCR program, I was able to receive financial support to complete my thesis project and mentorship support in cancer research. I am grateful to the OSCR program for my training and it has certainly helped me during my career as a resident as I work toward fellowship and my future career as a hematologist-oncologist and translational researcher.”- Andrew Gdowski, D.O., Ph.D., Resident Physician at The University of Texas at Austin Dell Medical School


CPRIT Trainee Aparna Gorthi, Ph.D.
The Cancer Research Program at The University of Texas Health Science Center at San Antonio (RP101491)
The Cancer Research Program, led by UT Health San Antonio’s Babatunde Oyajobi, M.D., Ph.D., trains undergraduate students to postgraduates with a curriculum that stresses both basic and translational research, providing the broad background in oncology needed for today’s research.
A key component of the training includes a strong mentorship network, with 59 funded mentors and faculty experienced in a broad range in cancer research including genomics/proteomics, cell signaling and receptor biology, DNA repair, metastasis, radiology, drug discovery, pediatric oncology, and carcinogenesis and prevention. The Cancer Research Program also introduces minority undergraduate students to research in a summer program where they can train with any of the program’s mentors.
Aparna Gorthi, Ph.D., post-doctoral fellow at Greehey Children’s Cancer Research Institute at UT Health San Antonio, participated in the Cancer Research Program. Dr. Gorthi, who has an undergraduate and master’s degree in engineering, completed her Ph.D. dissertation research focused on delineating the mechanism underlying PARP inhibitor sensitivity in Ewing sarcoma. She made several important discoveries and published her dissertation research in the journal Nature in 2018. As a postdoctoral trainee in the Department of Population Sciences, Dr. Gorthi contributed to other recent collaborative publications in Nature. This year the journal Cancers published computational validation of her earlier discoveries. In 2018, Dr. Gorthi received the prestigious American Association for Cancer Research (AACR)-Astra Zeneca Stimulating Therapeutic Advances through Research Training (START) grant totaling $225,000 over three years.
CPRIT Trainee Amit Guptra, Ph.D.
Computational Cancer Biomedicine Training Program at the University of Houston (RP140113)
This inter-institutional program, led by University of Houston’s James Briggs, Ph.D., utilizes the considerable resources and distinguished faculty of the six institutions of the Gulf Coast Consortia/Keck Center: University of Houston, Rice University, Baylor College of Medicine, The University of Texas MD Anderson Cancer Center, The University of Texas Health Science Center-Houston, and The University of Texas Medical Branch at Galveston. These institutions have a long-standing history of working together in undergraduate, graduate, and postdoctoral training programs, and each brings a unique research and/or clinical strength necessary for the success of this Computational Cancer Biology Training Program (CCBTP).
The CCBTP postdoctoral training program increases the number of skilled, multidisciplinary cancer researchers in Texas who are uniquely able to integrate the tools, ideas, and materials of computational science and biology. The complex interplay among the often disparate clinical and research computational science applications that impact cancer medicine and biology demands innovative and interdisciplinary methods of cancer research. Cancer biologists and clinicians must collaborate with leading researchers in mathematics, computational science and engineering to more effectively study cancer and develop cutting-edge tools that will lead to breakthroughs and creative solutions.
Amit Guptra, Ph.D., credits the CCBTP with preparing him for his chosen career path in computational chemisty. The interdisciplinary dual mentorship between The University of Texas Health Science Center at Houston and The University of Texas M.D. Anderson Cancer Center advanced Dr. Guptra’s computational skill set, while also giving him a deeper understanding of cancer biology. Monthly trainee meetings, seminars, grant writing workshops and sponsorships allowing him to present his work at national conferences improved Dr. Guptra’s interpersonal, communication and presentation skills, making him a more viable candidate for his current role as Computational Chemist at the Texas Medical Center’s Accelerator for Cancer Therapeutics.
CPRIT is building a vibrant life sciences and prevention infrastructure across the state and improving Texas’ national standing in both cancer research and the biomedical industry. Elements necessary to create and sustain this growing ecosystem include physical assets like core facilities, advanced manufacturing capabilities, state-of-the-art computational modeling resources, and accelerator hubs. Another crucial component is the collaborations CPRIT fosters across the state to tackle some of Texas’ most intractable cancers and reach more Texans with successful prevention projects proven to save lives and reduce the burden of cancer. By integrating unmatched biological, medical, chemical, and computational expertise with advanced technology, Texas is also priming the pipeline for new company development.
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the fastest-rising cause of cancer deaths nationwide and in Texas. Hispanics and African Americans in Texas are particularly affected due to an increased prevalence of HCC risk factors. CPRIT has committed $108 million over the past three years to mitigating HCC risk factors and finding better treatments.

Twelve exceptional HCC researchers brought to Texas with $37.4 million in CPRIT recruitment grants
In 2018, The University of Texas Southwestern Medical Center recruited Yujin Hoshida, M.D., Ph.D., from Icahn School of Medicine at Mount Sinai with a $4 million CPRIT Recruitment of Rising Stars Award (RR180016).
$55.4 million in investigator-initiated grants researching the state’s HCC crisis
Doctors diagnose HCC patients often when the cancer is already advanced and available standard treatment options may increase survival by only a few months. HCC patients desperately need new treatments. The University of Texas M.D. Anderson Cancer Center’s David Tweardy, M.D., has received multiple CPRIT grants (RP100421, RP110291, DP150069, and RP180473) to support the discovery, pre-clinical development, and clinical study of a novel treatment that targets a cancer-causing protein within liver cancers called STAT3.


Developing therapies for high-risk pediatric liver cancer
CPRIT’s $6 million multi-investigator research award (RP180674) links investigators studying biomarkers that show up in high-risk pediatric liver cancers at Baylor College of Medicine and The University of Texas Health Science Center at San Antonio. The goal of the project, led by Dolores Lopez-Terrada, M.D., Ph.D. at Texas Children’s Hospital, is to determine and evaluate new therapy targets and develop more effective and safer treatments.

A new statewide Collaborative Action Program to address the HCC problem in Texas
Hashem El-Serag, M.D., Baylor College of Medicine, leads the Texas Collaborative Center for Hepatocellular Cancer (TeCH) (RP190641) that helps scientists researching liver cancer work faster and better, team up with other researchers, and share discoveries with doctors, the community, and the public to change healthcare. Four other projects at Baylor College of Medicine (RP200633), The University of Texas M.D. Anderson Cancer Center (RP190513), and The University of Texas Southwestern Medical Center (RP200554) are studying ways to prevent HCC, detect HCC early, and reduce the HCC risk disparities.
CPRIT funds evidence-based prevention education, vaccinations, and screenings services to prevent and detect cancer early in underserved populations throughout Texas. Through steady investment over the past decade, CPRIT has created relationship infrastructure joining together networks of public health and community partners. The growing cancer prevention ecosystem in Texas is expanding the original footprint of successful CPRIT-funded programs to include more counties that do not have adequate access to cancer prevention services.
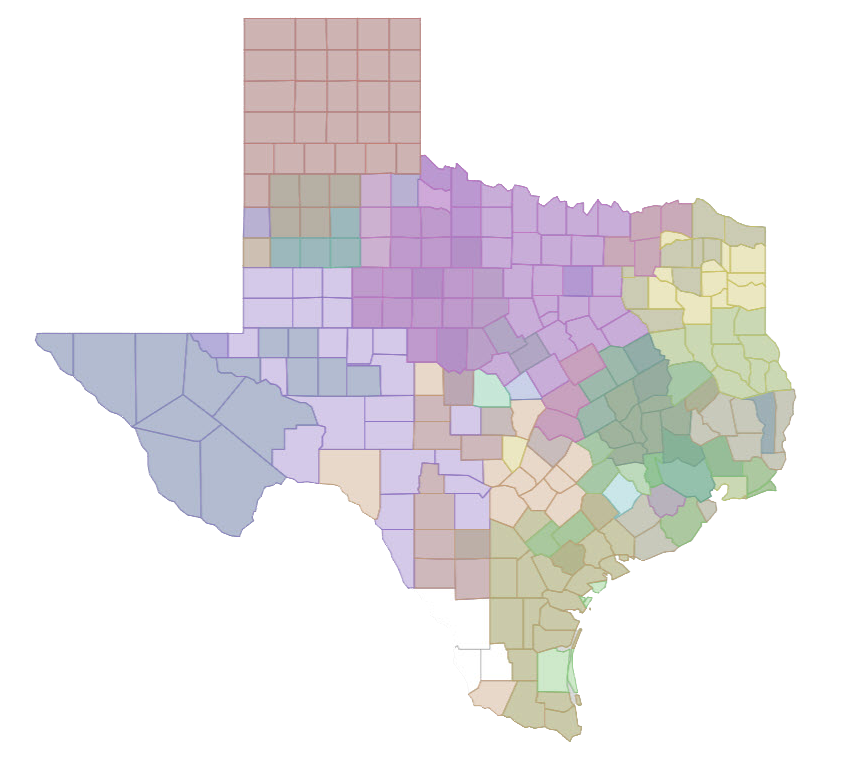
The map below dynamically illustrates the impact of expanding over time successful CPRIT-funded breast and colorectal cancer screening projects to additional rural and underserved counties in Texas. The map shows only the breast and colorectal cancer screening expansion projects active in FY 2020; this map shows all active prevention projects in FY 2020.
Instructions:
Click on a prevention program project ID to show counties served or click the play button on the timeline to show how the reach of the prevention project has expanded over the past decade.
The interactive map is only available on larger width devices. Try turning your device horizontally (landscape mode)
Cancer Site Selection
Texas is home to massive research institutions and top tier cancer research talent. Discoveries made here are a major driver of new cancer drugs, devices, and diagnostics. They also attract industry partners eager to develop leading-edge science. With CPRIT’s steady investments in the state’s academic research prowess, we are priming the pipeline for new companies that translate promising theories into groundbreaking therapeutics and stimulating industries like manufacturing and clinical research organizations that support advanced medical research, clinical trials and drug development. Cultivating the life science infrastructure across Texas supports job growth and generates tax revenues for the state.


The University of Texas M.D. Anderson Cancer Center and ImmunoGenesis: Immunotherapy discoveries at M.D. Anderson lead to a new company created to accelerate promising treatments
The immune system is the most powerful tool for actually eliminating cancer. There’s no magic bullet. But what there is, is a collection of bullets that, when put together, can eliminate cancer.
Immunotherapy drugs, called checkpoint inhibitors, have revolutionized therapy for some cancers by working in concert with the patient’s immune system T-cells to attack cancerous tumor cells. These drugs target “hot” tumors inflamed by the body’s T-cell response in cancers including melanomas, head and neck, bladder, kidney, and non-small cell lung cancer. Unfortunately, more than half of patients have cancers with “cold” tumors that do not activate an immune system response.
Michael Curran, Ph.D., and his team at The University of Texas M.D. Anderson Cancer Center are studying reasons for immune resistance in the “coldest” tumors, pancreatic and prostate adenocarcinoma and glioblastoma. Recognizing his innovative approach to a significant unmet need, CPRIT awarded Dr. Curran an Individual Investigator grant totaling $900,000 in February 2019 (RP190218). The CPRIT grant funded Dr. Curran’s research focused on the role of an immune checkpoint receptor, PD-L2, in supporting the growth of cancers that do not respond to existing immunotherapies. Dr. Curran used the CPRIT grant to complete preliminary studies showing his potential new treatment outperformed the most potent existing drug available to unleash the immune system against cancer.
Based on the information and data gained from his CPRIT-funded work, Dr. Curran founded ImmunoGenesis in 2019 to continue development of the new treatment, IMGS-001. The new company applied for and received a CPRIT Product Development award in August 2020 (DP200094). CPRIT’s $15 million investment provides the capital for the new company to complete preclinical work necessary for FDA approval and to begin clinical trials of IMGS-001 at M.D. Anderson and other clinical sites in Texas. The company also is developing a biomarker test to identify patients most likely to benefit from IMGS-001.
The ImmunoGenesis team sees the potential for the IMGS-001 treatment to benefit the majority (55%) of cancer patients not helped by current immunotherapies. CPRIT’s funding was critical to transforming Dr. Curran’s early idea into a data-backed approach ready for licensing and regulatory approval to test in patients. In addition to nurturing a promising advance in cancer treatment, CPRIT’s investment adds to the number of new oncology companies founded or relocated in Texas to accelerate development of promising university lab discoveries. This creates high quality new jobs and expands a growing industry in the state.


The University of Texas Southwestern Medical Center and Barricade Therapeutics: Discovery and advancement of novel first-in-class therapy to treat and prevent colorectal cancer
Colorectal cancer (CRC) is one of the most common cancers, affecting 1.8 million people each year. Despite the best therapies available today, fewer than one in 12 patients with advanced CRC survives for five years, and almost 900,000 people die from CRC annually.
CPRIT awarded Jerry W. Shay, Ph.D., and his team at The University of Texas Southwestern Medical Center a $400,000 Individual Investigator grant (RP130189) in December 2012 to study a class of molecules, named TASINs, he discovered to target an alteration of the adenomatous polyposis coli (APC) gene. This discovery could be key to catching CRC at its earliest stage, since more than 80% of CRC cases first present with a mutation to the APC. TASINs detect and kill cancer cells containing the mutated APC gene without harming normal cells – a process of “synthetic lethality.”
Building on his successful CPRIT work, in December 2015 Dr. Shay applied for and received a second Individual Investigator grant ( RP160180) for $900,000 to further investigate how the targeted TASIN therapy he developed with his first CPRIT grant works in mouse models of human tumors and in patient-derived primary tumors in xenografts.
Fort Worth-based Barricade Therapeutics was founded in 2017 to advance the discoveries made at UT Southwestern by Dr. Shay and his team. The next step in this development process is to identify the best TASIN molecule and complete the final FDA-mandated studies to begin human trials. Based on the strength of its scientific work and its innovative approach, Barricade received a $3 million CPRIT Product Development Seed Award in February 2020 (DP200056). Barricade’s experienced Texas-based drug development team plans to bring TASIN to market by 2026.
CPRIT’s overarching purpose is to reduce the physical, economic and emotional burden of cancer in Texas. The best way to do this is by stopping cancer before it takes hold or detecting it in its earliest stages when treatment is less toxic, less costly, and more successful. For those cancers that health care providers cannot stop or detect early, then CPRIT must make groundbreaking treatments and cures for cancer available faster. Innovation is the key to accomplishing this fundamental goal.
Advances in technology over the past decade have significantly enhanced scientists’ ability to dissect individual patients’ cancers down to the very genes that cause them to grow and progress. This opens new doors to the development of patient-specific treatment. But these groundbreaking cancer discoveries made in university laboratories across Texas are most valuable when scientists and drug developers can translate those findings into new therapies available to cancer patients.
An important and necessary component of CPRIT’s mission to develop innovative cancer treatments is supporting clinical study research conducted by Texas-based companies and research institutions. Precision cancer medicine requires tests or biomarkers to determine the genetic and protein drivers of a given cancer and new drugs that effectively target and block those drivers. Some examples below show how CPRIT’s investment in companies undertaking clinical trials is crucial to accelerating the advancement of promising drug candidates.

A promising therapy with fewer side effects for brain cancer patients is now preparing for Phase 3 trials. Medicenna Therapeutics Inc. is developing treatments for brain cancers that affect both adults and children, including recurrent glioblastoma multiforme (rGBM). The company received a $14.1 million Product Development award in 2015 (DP150031) to develop and test MDNA55, a novel immunotherapy to treat rGBM and other brain cancers. After five years of work, Medicenna announced October 15, 2020 that the U.S. Food and Drug Administration advised the company to proceed with an open-label hybrid control design for a Phase 3 registration trial of MDNA55 in rGBM patients.
GBM cancers make a protein on the cancer cells’ surface called the IL-4 receptor (IL-4R). Since most normal cells have no IL-4R, MDNA55 works as an IL4-guided toxin. Clinicians have studied MDNA55 in five clinical trials involving 132 patients, including 112 adults with rGBM.
The Houston and Toronto-based company successfully completed its Phase 2b trial of MDNA55 and announced the results at the 2020 American Society of Clinical Oncology Virtual Annual Meeting on May 29, 2020. Highlights from the ASCO presentation included a comparison of MDNA55 with an eligibility-matched Synthetic Control Arm, which demonstrated an improvement in median overall survival of 61%. When stratified by IL4R status, IL4R High subjects in the MDNA55 arm demonstrated improved median overall survival by 155%. This is positive news because rGBM is the most common and uniformly fatal form of adult brain cancer.
Medicenna’s Phase 3 registration trial will compare the new drug to the standard of care to assess the side effects and determine whether the new drug improves patient outcomes over the standard of care. This trial generates the data necessary to receive approval from the U.S. Food and Drug Administration as a new drug.
The FDA has already approved Fast-Track and Orphan Drug status for MDNA55. Fast Track is an FDA-designed process to expedite the development and review of new drugs with the potential to treat serious or life-threatening conditions and fill unmet medical needs. The process streamlines regulatory submissions and enables more frequent communications with the agency to resolve questions quickly which often leads to earlier drug approval and access by patients.

Developing and testing a novel drug for rare childhood cancer in Phase 1/2 trials. Salarius Pharmaceuticals is a clinical-stage biotechnology company targeting cancers caused by mis-regulated gene expression. CPRIT’s investment supports the company’s current single-arm Phase 1/2 clinical trial of its lead drug candidate, Seclidemstat, in up to 50 patients at eight sites throughout the country, including a trial site at The University of Texas M.D. Anderson Cancer Center. Houston-based Salarius specializes in developing novel drugs for rare pediatric cancers and other cancers by focusing on treatments that interrupt the final steps of the signaling cascade.
The company received a CPRIT Product Development Award in 2016 (DP160014) totaling up to $18.7 million to develop and test Seclidemstat in a Phase 1/2 (dose escalation and dose expansion) study for refractory or relapsed Ewing sarcoma. Ewing sarcoma is a rare and deadly pediatric bone cancer for which there are no approved targeted treatments. Seclidemstat targets the Lysine Specific Histone Demethylase 1 pathway (LSD1), a cellular control protein that is overactive in a range of cancers, including Ewing sarcoma. In August 2019, the company announced that it will also test Seclidemstat for the treatment of glioblastoma in a collaboration with the Ivy Brain Tumor Center at the Barrow Neurological Institute.
In recognition of the high unmet medical need facing Ewing’s sarcoma patients, the U.S. Food and Drug Administration granted Seclidemstat a Fast-Track Designation, as well as Orphan and Rare Pediatric Disease Designations, to treat patients with Ewing sarcoma who have relapsed or are refractory to standard-of-care therapy. Fast Track is an FDA-designed process to expedite the development and review of new drugs with the potential to treat serious or life-threatening conditions and fill unmet medical needs. The process streamlines regulatory submissions and enables more frequent communications with the agency to resolve questions quickly which often leads to earlier drug approval and access by patients.

Accelerating treatments that enhance the potential of immunotherapy in Phase 1 trials. San Antonio-based Pelican Therapeutics treated its first patient in June 2020 in the company’s Phase 1 clinical trial of PTX-35. The company’s first-in-human Phase 1 trial will enroll up to 30 patients with advanced solid tumors not responding to standard-of-care treatments. PTX-35 is a novel, first-in-class drug designed to treat multiple solid tumor types. Preclinical studies demonstrate PTX-35, in combination with antigen-driven immunotherapies, helps eliminate tumors. Pelican received a $15.2 million CPRIT Product Development award in 2016 (DP160012) to support pre-clinical development, manufacturing, and Phase 1 clinical study of PTX-35.
Immunotherapy has enormous potential to improve survival, and even cure, patients with many types of cancer. Unfortunately, clinical responses to these drugs only occur in a minority of patients. In patients that do respond, researcher have observed strong associations between the degree of CD8+ cytotoxic T-cell activation and the strength of the anti-tumor immune response. The CD8+ is the class of T-cell responsible for eliminating tumor cells in patients. Pelican is studying how combination immunotherapeutics providing specific stimulation of CD8+ T-cells can lead to better patient outcomes.
Pelican Therapeutics is developing the immunotherapy agent for cancer patients. Early studies indicate that it is a best-in-class product due to specific activation of killer T-cells, the strongest predictor of survival benefit in cancer immunotherapy.
With the CPRIT award, Pelican has finalized development and will complete the clinical trial studying the novel drug in several types of solid tumor cancers, including patients with lymphoma, lung, prostate, pancreatic and ovarian cancer. Its first clinical trial site is in San Antonio.
Although the excitement and challenge associated with clinical cancer research has never been greater, there is a decline in exceptionally talented individuals entering a career in cancer clinical investigation. One reason for this decline is the growing demand to generate clinical revenue, leaving clinical faculty with fewer opportunities to pursue research. The burden of medical school debt also limits a trainee’s options for extending training and pursuing an academic career. The increasingly complex nature of cancer clinical research, which requires specialized training not offered by clinical training programs, also steers promising clinical faculty from a career as a clinical investigator.
CPRIT developed a new grant award in FY 2020 to replenish the pipeline of clinical investigators conducting innovative clinical trials at Texas institutions. The five-year award supports an early-career cancer physician with salary support and backs the development of clinical research skills needed to conduct patient-oriented clinical studies. The inaugural members of the CPRIT’s 2020 Class of Early Clinical Investigators include:

Premal Lulla, M.D, Baylor College of Medicine
Dr. Lulla is a member of the stem cell transplantation program at Baylor College of Medicine, where he treats patients with hematologic malignancies. He plans to use his award (RP200584) to bring a novel T-cell therapy developed at Baylor to a clinical trial for patients undergoing stem cell treatment of acute myelogenous leukemia

David Hsieh, M.D., The University of Texas Southwestern Medical Center
Dr. Hsieh plans a clinical trial of a novel approach to augmenting the responses of liver cancers to checkpoint immunotherapies (RP200549). CPRIT-funded clinical and translational investigators at UT Southwestern, including Dr. David Gerber and CPRIT Scholar Dr. Hao Zhu, will mentor Dr. Hsieh.

Nicolas Palaskas, M.D., The University of Texas M.D. Anderson Cancer Center
Dr. Palaskas is a clinical cardiologist specializing in the new subspecialty of cardio-oncology, which involves the diagnosis and treatment of cancer and cancer treatment-related heart disease. He plans clinical trials designed to mitigate the cardiac toxicity associated with novel cancer immune therapies (RP200670).

Chad Tang, M.D., The University of Texas M.D. Anderson Cancer Center
Dr. Chad Tang is a radiation oncologist who is conducting a CPRIT-supported trial to investigate the efficacy of radiation therapy to manage low volume metastatic cancer (RP200669). The ECI award will allow him to emerge as a national leader in this innovative approach.
Including cervical cancer, high risk types of the human papillomavirus (HPV) cause at least six different kinds of cancer in men and women and were responsible for 36,000 cancer diagnoses in 2019. Scientists have developed a highly effective vaccine against the HPV virus, but despite its proven ability to prevent cancer, many parts of Texas have HPV vaccination rates much lower than the national average. For example, only 18 percent of eligible young women receiving care in 2018 at The University of Texas Medical Branch’s gynecology clinics in McAllen and Galveston report receiving the vaccine. The low vaccination rate is concerning because many clinic patients are low-income or members of racial or ethnic groups with higher rates of HPV-related cancer cases and deaths.
Innovation is important not only in scientific development of life saving vaccines, but also necessary to ensure that enough people are benefiting from the proven cancer prevention strategy. CPRIT funds several prevention projects that use inventive approaches to increase the number of young Texans fully vaccinated against HPV. Strategies include patient navigation services, partnering with schools, no-cost vaccinations, provider education, and innovative reminder methods such as text messaging to complete the full series. The tactics are working to significantly increase vaccination rates across Texas, which will pay dividends in cancer cases avoided.
Project ID:
PP160080
Awarded Amount: $1,302,955
Project Title:
Promoting HPV vaccination among Hispanic adolescents and young adults using Health Care System-Based Interventions and Community Outreach
Hispanic women in Hidalgo County have higher cervical cancer incidence and mortality rates compared to Hispanic women nationally. Although the HPV vaccine can prevent cervical cancer and other cancers associated with HPV, the proportion of Hispanic teenagers in Texas aged 13-17 receiving at least one dose of the HPV vaccine is at least 10% lower compared to Hispanic teenagers nationwide.
Dr. Daisy Morales-Campos at The University of Texas at Austin leads the project to increase HPV vaccination among underserved, predominantly low-income Hispanic adolescent and young adult patients in four primary care practices in Hidalgo County. The project includes five collaborative partners - the Institute for Health Promotion Research; Lower Rio Grande Valley Area Health Education Center; Nuestra Clinica del Valle; Texas A&M University’s Colonias Program; and The Texas Immunization Partnership. Together these partners innovatively combine multiple, evidence-based strategies and build upon existing partnerships and infrastructure between academic, public and private health clinics, and community stakeholders to increase HPV vaccine initiation and completion rates.
Project ID:
PP180080
Awarded Amount: $1,019,690
Project Title:
HPV Vaccination in a Pediatric Minority-Based Community Oncology Network
Childhood cancer survivors have significantly higher rates of HPV-related malignancies (40% more in females and 150% more in males). Despite this, they have extremely low HPV vaccination rates. Of the childhood cancer survivors treated at The University of Texas Health Science Center at San Antonio, only 13.5% of those vaccine-eligible initiated the HPV vaccine and 6% completed the series.
This project, led by Dr. Allison Grimes at UT Health San Antonio, focuses on increasing HPV vaccination rates among children served by the South Texas Pediatric Minority Underserved NCI Community Oncology Research Program (STPMU NCORP). The STPMU NCORP network operates as a consortium of five regional pediatric institutions: UT Health San Antonio, Methodist Children’s Hospital (San Antonio), Dell Children’s Hospital (Austin), Driscoll Children’s Hospital (Corpus Christi), and Texas Tech University Health Sciences Center at El Paso. Collectively, the STPMU NCORP institutions follow more than 1,480 pediatric cancer survivors across the state, most of whom will be age-eligible for the HPV vaccine.
Project IDs:
PP190058
and
PP140211
Awarded Amount: $3,462,976
Project Title:
Tiempo de Vacunarte (Time to Get Vaccinated)
The project, originally targeting El Paso County in 2014, expanded to serve a total population of 863,000 people in 2019 with the inclusion of Hudspeth, Culberson, Presidio, and Brewster Counties. People living in the Texas-Mexico border counties suffer from a higher incidence and mortality for HPV-associated cancers, including cervical cancer for Hispanic women and penile cancer for Hispanic men. Compounding this health disparity, the area does not have enough physicians for its population. Initiation rates of HPV vaccination are quite low, particularly among young adults between the ages of 18 and 34, and completion rates are negligible for this age range.
Dr. Jennifer Molokwu of Texas Tech University Health Sciences Center at El Paso leads this culturally tailored program focusing on individuals aged 9-26 years old through outreach, education, navigation, and clinical services. The project partners with community sites including school districts, community centers, clinics, faith-based organizations, food banks, city/county services, local and state health departments, and other community-based organizations.
Project ID:
PP190041
Awarded Amount: $299,966
Project Title:
Adolescent Vaccination Program: Online Decision Support for Adoption of Evidence-based HPV Vaccination Strategies by Texas Pediatric Clinics
Researchers know certain evidence-based strategies - including electronic decision reminders, health care provider training on message bundling and patient interaction, and direct education for patients - increase HPV vaccination rates. Despite this, adoption of these strategies in clinical networks has been piecemeal because many lack the expertise and resources to adopt, implement, and maintain a systems approach to HPV vaccination.
The project, led by Dr. Ross Shegog at The University of Texas Health Science Center at Houston, develops the web-based Adolescent Vaccination Program Implementation Tool (AVP-IT) to support the adoption, implementation, and maintenance of a suite of evidence-based HPV vaccination strategies in pediatric clinics throughout Texas. The AVP-IT builds on a successful CPRIT-funded prevention collaborative project between UTHealth Houston, Baylor College of Medicine, and Texas Children’s Pediatric Practice Network in Houston (PP180089), which used a suite of proven strategies to increase HPV vaccination rates in a large urban pediatric clinical practice from 53.9% in 2015 to 76.9% in 2017.
Project ID:
PP160010
Awarded Amount: $1,409,909
Project Title:
Maximizing opportunities for HPV vaccination in the Golden Triangle
HPV vaccination has a direct impact on public health and cancer prevention, but vaccination rates are particularly low in Texas’ Golden Triangle (Jefferson and Orange Counties). This is concerning because cervical cancer occurs twice as often and oropharyngeal cancer in men nearly 1.5 times more often in Orange County than the rest of Texas.
This project, led by The University of Texas Medical Branch at Galveston’s Dr. Abbey Berenson, uses multiple strategies to reduce barriers and helps adolescents and young adults in East Texas easily obtain this highly effective vaccine. Strategies to increase the number vaccinated include patient navigation services, vaccination at no cost to the patient, thorough patient tracking, electronic (texting) reminder methods, provider education, and targeted social media outreach. Clinicians will encourage patients to refer their partners to increase the number of males vaccinated.
Project ID:
PP190004
Awarded Amount: $2,499,411
Project Title:
Partnering with schools and clinics to expand a highly successful HPV vaccination program for 9-17 year olds from Medically Underserved Areas
This project builds upon the extraordinarily successful CPRIT-funded project (PP150004) that vaccinated 2,473 boys and girls seen in three UTMB pediatric clinics in Galveston and Brazoria counties; an impressive 99% of these adolescents completed the series, well surpassing national averages. The University of Texas Medical Branch at Galveston’s Dr. Abbey Berenson leads this expansion project, which extends coverage to the UTMB clinics serving patients in 23 other counties where more than 75% of age-eligible adolescents had not initiated the HPV vaccination series.
In addition to expanding pediatric clinics to family practice clinics, Dr. Berenson’s team is adding onsite vaccination at eight schools in rural counties that lack a health department. This combined approach will more than double the number of doses given during the original project. The project includes a significant professional education component to reduce barriers to HPV vaccination, establishing unique partnerships with the UTMB medical, nursing, and health professions schools, three county medical societies and major Texas professional societies for pediatricians, gynecologists and school nurses.
Dr. Abbey Berenson is the program director for eight successful CPRIT prevention projects using innovative opportunities to increase cervical cancer screenings and HPV vaccinations in underserved populations in Texas. In this video, she discusses some of her CPRIT-funded projects.